Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Ir M
Ir M ssbauer spectroscopic parameters of Vaska's complexes and their oxidative adducts
ssbauer spectroscopic parameters of Vaska's complexes and their oxidative adductsKaneko, Masashi; Nakashima, Satoru*
Inorganic Chemistry, 60(17), p.12740 - 12752, 2021/09
Times Cited Count:3 Percentile:31.46(Chemistry, Inorganic & Nuclear)In the present study, density functional theory (DFT) calculation was applied to Vaska's complexes of formula  -[IrCl(CO)(PPh
-[IrCl(CO)(PPh )
) ], and their oxidative adducts with small molecules (YZ) including H
], and their oxidative adducts with small molecules (YZ) including H , i.e.,
, i.e.,  -[IrCl(YZ)(CO))(PPh
-[IrCl(YZ)(CO))(PPh )
) ], to successfully correlate the electronic states of the complexes with the corresponding
], to successfully correlate the electronic states of the complexes with the corresponding  Ir M
Ir M ssbauer spectroscopic parameters. After confirming the reproducibility of the DFT methods for elucidating the equilibrium structures and
ssbauer spectroscopic parameters. After confirming the reproducibility of the DFT methods for elucidating the equilibrium structures and  Ir M
Ir M ssbauer isomer shifts of the octahedral Ir complexes, the isomer shifts and quadrupole splitting values of Vaska's complexes and their oxidative adducts were calculated. A bond critical point analysis revealed that the tendency in the isomer shifts was correlated with the strength of the covalent interaction in the coordination bonds. In an electric field gradient (EFG) analysis of the oxidative adducts, the sign of the principal axis was found to be positive for the complex with YZ = Cl
ssbauer isomer shifts of the octahedral Ir complexes, the isomer shifts and quadrupole splitting values of Vaska's complexes and their oxidative adducts were calculated. A bond critical point analysis revealed that the tendency in the isomer shifts was correlated with the strength of the covalent interaction in the coordination bonds. In an electric field gradient (EFG) analysis of the oxidative adducts, the sign of the principal axis was found to be positive for the complex with YZ = Cl and negative for the complex with YZ = H
and negative for the complex with YZ = H . This reversal of the sign of the EFG principal axis was caused by the difference in the electron density distribution for the coordination bonds between Ir and YZ, according to a density of states analysis.
. This reversal of the sign of the EFG principal axis was caused by the difference in the electron density distribution for the coordination bonds between Ir and YZ, according to a density of states analysis.
 complexes of mixed N,O-donor ligands demonstrated on a 10-fold [Eu(TPAMEN)]
complexes of mixed N,O-donor ligands demonstrated on a 10-fold [Eu(TPAMEN)] chelate complex
chelate complexSchnaars, K.; Kaneko, Masashi; Fujisawa, Kiyoshi*
Inorganic Chemistry, 60(4), p.2477 - 2491, 2021/02
Times Cited Count:6 Percentile:57.56(Chemistry, Inorganic & Nuclear)To reduce high-level radiotoxic waste generated by nuclear power plants, highly selective separation agents for minor actinides are mandatory. The mixed N,O-donor ligand 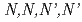 -tetrakis[(6-carboxypyridin-2-yl)methyl]ethylenediamine (H
-tetrakis[(6-carboxypyridin-2-yl)methyl]ethylenediamine (H TPAEN) has shown good performance as a masking agent in Am
TPAEN) has shown good performance as a masking agent in Am /Eu
/Eu separation studies. In this work, we examine whether a decrease in O-donor basicity can promote the M
separation studies. In this work, we examine whether a decrease in O-donor basicity can promote the M -N
-N interactions. Therefore, we replace the deprotonated "charged" carboxylic acid groups of TPAEN
interactions. Therefore, we replace the deprotonated "charged" carboxylic acid groups of TPAEN by neutral amide groups and introduce
by neutral amide groups and introduce 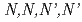 -tetrakis[(6-
-tetrakis[(6-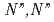 -diethylcarbamoylpyridin-2-yl)methyl]ethylenediamine (TPAMEN) as a new ligand. TPAMEN was crystallized with Eu(OTf)
-diethylcarbamoylpyridin-2-yl)methyl]ethylenediamine (TPAMEN) as a new ligand. TPAMEN was crystallized with Eu(OTf) and Eu(NO
and Eu(NO )
) 6H
6H O to form positively charged 1:1 [Eu(TPAMEN)]
O to form positively charged 1:1 [Eu(TPAMEN)] complexes in the solid state. Alterations in the M-O/N bond distances are compared to [Eu(TPAEN)]
complexes in the solid state. Alterations in the M-O/N bond distances are compared to [Eu(TPAEN)] and investigated by DFT calculations to expose the differences in charge/energy density distributions at europium(III) and the donor functionalities of the TPAEN
and investigated by DFT calculations to expose the differences in charge/energy density distributions at europium(III) and the donor functionalities of the TPAEN and TPAMEN. On the basis of estimations of the bond orders, atomic charges spin populations, and density of states in the Eu and potential Am and Cm complexes, the specific contributions of the donor-metal interaction are analyzed. The prediction of complex formation energy differences for the [M(TPAEN)]
and TPAMEN. On the basis of estimations of the bond orders, atomic charges spin populations, and density of states in the Eu and potential Am and Cm complexes, the specific contributions of the donor-metal interaction are analyzed. The prediction of complex formation energy differences for the [M(TPAEN)] and [M(TPAMEN)]
and [M(TPAMEN)] (M
(M = Eu
= Eu , Am
, Am ) complexes provide an outlook on the potential performance of TPAMEN in Am
) complexes provide an outlook on the potential performance of TPAMEN in Am /Eu
/Eu separation.
separation.
 O)
O) ]
] with nitrate ions by using density functional theory calculation
with nitrate ions by using density functional theory calculationKato, Akane*; Kaneko, Masashi; Nakashima, Satoru*
RSC Advances (Internet), 10(41), p.24434 - 24443, 2020/06
Times Cited Count:6 Percentile:31.01(Chemistry, Multidisciplinary)Complexation reactions of ruthenium-nitrosyl complexes in HNO solution were investigated by density functional theory (DFT) calculations in order to predict the stability of Ru species in high-level radioactive liquid waste (HLLW) solution. Equilibrium structure of [Ru(NO)(NO
solution were investigated by density functional theory (DFT) calculations in order to predict the stability of Ru species in high-level radioactive liquid waste (HLLW) solution. Equilibrium structure of [Ru(NO)(NO )
) (H
(H O)
O) ] obtained by DFT calculations reproduced the experimental Ru-ligands bond lengths and IR frequencies reported previously. Comparison of the Gibbs energies among the geometrical isomers revealed that the complexation reactions of the ruthenium-nitrosyl complexes with NO
] obtained by DFT calculations reproduced the experimental Ru-ligands bond lengths and IR frequencies reported previously. Comparison of the Gibbs energies among the geometrical isomers revealed that the complexation reactions of the ruthenium-nitrosyl complexes with NO
 proceed via the NO
proceed via the NO
 coordination to the equatorial plane toward the Ru-NO axis. We also estimated Gibbs energy differences on the stepwise complexation reactions to succeed in reproducing the fraction of Ru-NO species in 6 M HNO
coordination to the equatorial plane toward the Ru-NO axis. We also estimated Gibbs energy differences on the stepwise complexation reactions to succeed in reproducing the fraction of Ru-NO species in 6 M HNO solution, such as in HLLW, by considering the association energy between the Ru-NO species and the substituting ligands. Electron density analyses of the complexes indicated that the strength of the Ru-ligands coordination bonds depends on the stability of the Ru species and the Ru complex without NO
solution, such as in HLLW, by considering the association energy between the Ru-NO species and the substituting ligands. Electron density analyses of the complexes indicated that the strength of the Ru-ligands coordination bonds depends on the stability of the Ru species and the Ru complex without NO
 at the axial position is more stable than that wit NO
at the axial position is more stable than that wit NO
 , which might attribute to the difference in the trans influence between H
, which might attribute to the difference in the trans influence between H O and NO
O and NO
 . Finally, we demonstrated the complexation kinetics in the reactions
. Finally, we demonstrated the complexation kinetics in the reactions  . The present study is expected to enable us to model the precise complexation reactions of platinum-group metals in HNO
. The present study is expected to enable us to model the precise complexation reactions of platinum-group metals in HNO solution.
solution.
 Ru M
Ru M ssbauer parameters of ruthenium-nitrosyl complexes
ssbauer parameters of ruthenium-nitrosyl complexesKaneko, Masashi; Kato, Akane*; Nakashima, Satoru*; Kitatsuji, Yoshihiro
Inorganic Chemistry, 58(20), p.14024 - 14033, 2019/10
Times Cited Count:12 Percentile:62.96(Chemistry, Inorganic & Nuclear)We applied density functional theory calculations to ruthenium-nitrosyl complexes, which are known to exist in high-level radioactive waste, to give a theoretical correlation between  Ru M
Ru M ssbauer spectroscopic parameters (
ssbauer spectroscopic parameters ( and
and 
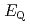 ) and ligand field strength (
) and ligand field strength (
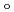 ) for the first time. The structures of the series of complexes, [Ru(NO)L
) for the first time. The structures of the series of complexes, [Ru(NO)L ] (L = Br
] (L = Br , Cl
, Cl , NH
, NH , CN
, CN ), were modeled based on the corresponding single-crystal X-ray coordinates. The comparisons of the geometries and total energies between the different spin states suggested that the singlet spin state of [Ru(II)(NO
), were modeled based on the corresponding single-crystal X-ray coordinates. The comparisons of the geometries and total energies between the different spin states suggested that the singlet spin state of [Ru(II)(NO )L
)L ] complexes were the most stable. The calculated results of both the
] complexes were the most stable. The calculated results of both the  and
and 
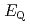 values reproduced the experimental results by reported previously and increased in the order of L = Br
values reproduced the experimental results by reported previously and increased in the order of L = Br , Cl
, Cl , NH
, NH , CN
, CN . Finally, we estimated the ligand field strength (
. Finally, we estimated the ligand field strength (
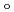 ) based on molecular orbitals, assuming C
) based on molecular orbitals, assuming C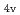 symmetry and showed the increase of
symmetry and showed the increase of 
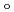 values in that order, being consistent with well-known spectrochemical series of ligands. The increase attributes to the strengthening of the abilities of
values in that order, being consistent with well-known spectrochemical series of ligands. The increase attributes to the strengthening of the abilities of  -donor and
-donor and 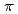 -acceptor of the L-ligands to the Ru atom, resulting in the increase of the
-acceptor of the L-ligands to the Ru atom, resulting in the increase of the  values.
values.
Nakashima, Satoru*; Kaneko, Masashi; Yoshinami, Keisuke*; Iwai, Saki*; Dote, Haruka*
Hyperfine Interactions, 239(1), p.39_1 - 39_15, 2018/12
Times Cited Count:2 Percentile:65.67(Physics, Atomic, Molecular & Chemical)The present study reveals the on/off of spin-crossover (SCO) phenomenon in assembled Fe(II) complexes bridged by bis(pyridyl) type ligand. Whether SCO phenomenon occurs or not in assembled Fe(II) complexes bridged by bis(pyridyl) type ligand is determined by local structure around iron atom. SCO phenomenon occurs when the coordinating pyridines facing to each other across the iron atom are propeller type, while the phenomenon does not occur when they are parallel type or distorted propeller type. DFT calculation explained that, in the shortening of Fe-pyridine bonds when changing from high-spin state to low-spin state, the pyridines of propeller type can approach the iron atom with smaller steric hindrance than those of parallel and distorted propeller type complexes. The local structure is controlled by introducing methyl substituent and introducing 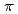 -system, changing SCO phenomenon. And the transition temperature of SCO is also controlled in assembled complexes bridged by 1,2-bis(4-pyridyl)ethane by mixing anionic ligand.
-system, changing SCO phenomenon. And the transition temperature of SCO is also controlled in assembled complexes bridged by 1,2-bis(4-pyridyl)ethane by mixing anionic ligand.
 Re
ReHashimoto, Kazuyuki; Wan, K. W. H. B. B.*; Matsuoka, Hiromitsu
Journal of Nuclear and Radiochemical Sciences, 6(3), p.193 - 196, 2005/12
The radioisotopes of rhenium ( Re and
Re and  Re) are attractive radionuclides for radiotherapy because of their energetic beta particles and gamma rays suitable for imaging. Mercaptoacetyltriglycine, MAG3 (N
Re) are attractive radionuclides for radiotherapy because of their energetic beta particles and gamma rays suitable for imaging. Mercaptoacetyltriglycine, MAG3 (N S ligand), is a useful bifunctional ligand in labeling monoclonal antibodies with metallic radionuclides. In this study, the labeling of MAG3 with carrier-free
S ligand), is a useful bifunctional ligand in labeling monoclonal antibodies with metallic radionuclides. In this study, the labeling of MAG3 with carrier-free  Re from a
Re from a  W/
W/ Re generator was investigated in detail. The
Re generator was investigated in detail. The  Re-MAG3 complex was synthesized by the direct labeling method and by the indirect labeling method using a transfer ligand (citric acid or gluconic acid). The dependence of the labeling yield upon the reaction conditions such as the concentrations of tin(II) chloride dihydrate as a reducing agent, S-benzoyl MAG3 and the transfer ligand, pH, temperature, reaction time and the addition of a carrier was examined. The labeling yield of
Re-MAG3 complex was synthesized by the direct labeling method and by the indirect labeling method using a transfer ligand (citric acid or gluconic acid). The dependence of the labeling yield upon the reaction conditions such as the concentrations of tin(II) chloride dihydrate as a reducing agent, S-benzoyl MAG3 and the transfer ligand, pH, temperature, reaction time and the addition of a carrier was examined. The labeling yield of  Re-MAG3 synthesized by the all method was over 90% under the optimum conditions.
Re-MAG3 synthesized by the all method was over 90% under the optimum conditions.
Ishimori, Kenichiro*; Watanabe, Masayuki; Kimura, Takaumi; Yaita, Tsuyoshi; Yamada, Takashi*; Kataoka, Yumiko*; Shinoda, Satoshi*; Tsukube, Hiroshi*
Chemistry Letters, 34(8), p.1112 - 1113, 2005/08
Times Cited Count:13 Percentile:44.74(Chemistry, Multidisciplinary)Stereochemical substitution on tripod ligand significantly offered efficient separation of trivalent actinides from trivalent lanthanides. Liquid-liquid extraction using chiral tris(2-pyridylmethyl)amine ligands as an extractant exhibited high efficiency and selectivity for trivalent actinides. A combination of chiral ligand and 2-bromodecanoic acid further enhanced extraction performance for trivalent actinides.
Fukushi, Keisuke*; Sato, Tsutomu*; Yanase, Nobuyuki; Minato, Junichi*; Yamada, Hirohisa*
American Mineralogist, 89(11-12), p.1728 - 1734, 2004/11
The sorption mechanism of As(V) on schwertmannite was investigated by both a batch sorption experiment and crystallographic considerations. The batch experiment was carried out as a function of As(V) concentration in acidic solution at 25  C. Crystallographic considerations indicate surface sites of schwertmannite comprise varied surface oxygen (hydroxyl) and SO
C. Crystallographic considerations indicate surface sites of schwertmannite comprise varied surface oxygen (hydroxyl) and SO groups. Sorption experiments showed reactive surface sites for As(V) sorption are surface SO
groups. Sorption experiments showed reactive surface sites for As(V) sorption are surface SO groups. As(V) sorption mechanism involves ligand exchange with solid phase SO
groups. As(V) sorption mechanism involves ligand exchange with solid phase SO . Results also suggest monodentate As(V) coordination with surface adsorbed SO
. Results also suggest monodentate As(V) coordination with surface adsorbed SO sites and bidentate As(V) coordination in structural originated SO
sites and bidentate As(V) coordination in structural originated SO sites. Estimated equilibrium constant for ligand exchange reaction describes the observed As(V) sorption behavior. The surface structure approach in this study reveals reactive surface sites in As(V) sorption on schwertmannite comprise surface SO
sites. Estimated equilibrium constant for ligand exchange reaction describes the observed As(V) sorption behavior. The surface structure approach in this study reveals reactive surface sites in As(V) sorption on schwertmannite comprise surface SO group instead of surface hydroxyl groups identified by former views.
group instead of surface hydroxyl groups identified by former views.
Ogawa, Kazuma*; Mukai, Takahiro*; Arano, Yasushi*; Hanaoka, Hirofumi*; Hashimoto, Kazuyuki; Nishimura, Hiroshi*; Saji, Hideo*
Journal of Labelled Compounds and Radiopharmaceuticals, 47(11), p.753 - 761, 2004/11
Times Cited Count:29 Percentile:61.59(Biochemical Research Methods)no abstracts in English
 in the presence of organic ligands
in the presence of organic ligandsYoshida, Takahiro; Suzuki, Yoshinori*; Ozaki, Takuo; Onuki, Toshihiko; Francis, A. J.*
Proceedings of International Symposium on Radioecology and Environmental Dosimetry, p.296 - 300, 2004/03
The effects of organic ligands (citric acid, desferrioxamine B (DFO) and ethylenediaminetetraacetic acid (EDTA)) on Eu(III) adsorption by an aerobic bacterium  were investigated. After incubation of
were investigated. After incubation of  with 2
with 2  M Eu(III) without organic ligands in a 0.1 M NaCl solution containing 3.9 mg
M Eu(III) without organic ligands in a 0.1 M NaCl solution containing 3.9 mg /L biomass for 1 hour, over 90% Eu(III) was adsorbed on the bacterial cells at pH 4 - 7. Eu(III) adsorption by bacterial cells was depleted at pH 4 - 7 when equimolar EDTA was present, while citric acid and DFO showed less effect on the depletion of Eu(III) adsorption. X-ray photoelectron spectroscopy (XPS) indicated that the adsorbed Eu(III) on cells was covalently bound to carboxyl and/or hydroxyl functional groups of the cell surface. These results suggest that carboxyl and/or hydroxyl functional groups of cell surface show a higher affinity with Eu(III) than citric acid and DFO and have the potential to prevent migration of Eu-organic ligands complexes with low stability constants in environments.
/L biomass for 1 hour, over 90% Eu(III) was adsorbed on the bacterial cells at pH 4 - 7. Eu(III) adsorption by bacterial cells was depleted at pH 4 - 7 when equimolar EDTA was present, while citric acid and DFO showed less effect on the depletion of Eu(III) adsorption. X-ray photoelectron spectroscopy (XPS) indicated that the adsorbed Eu(III) on cells was covalently bound to carboxyl and/or hydroxyl functional groups of the cell surface. These results suggest that carboxyl and/or hydroxyl functional groups of cell surface show a higher affinity with Eu(III) than citric acid and DFO and have the potential to prevent migration of Eu-organic ligands complexes with low stability constants in environments.
Watanabe, Masayuki; Nankawa, Takuya*; Yamada, Teppei*; Kimura, Takaumi; Namiki, Kosuke*; Murata, Masaki*; Nishihara, Hiroshi*; Tachimori, Shoichi
Inorganic Chemistry, 42(22), p.6977 - 6979, 2003/11
Times Cited Count:34 Percentile:75.23(Chemistry, Inorganic & Nuclear)A tripodal ligand, tris(2-pyridyl)carbinol affords a novel tetradentate coordination mode in homodinuclear lanthanide complexes, which exhibit remarkably short distance between the metal ions. Strong fluorescence of Eu(III) and Tb(III) complexes with the ligand demonstrate that the ligand has a suitable excited state for energy transfer from the ligand to Eu(III) and Tb(III) center.
Fukushi, Keisuke*; Sato, Tsutomu*; Yanase, Nobuyuki
Environmental Science & Technology, 37(16), p.3581 - 3586, 2003/07
Times Cited Count:71 Percentile:79.84(Engineering, Environmental)The mechanism of As(V) sorption on schwertmannite was investigated by a batch sorption experiment as a function of solution As(V) concentration under acidic conditions (pH 3.3  0.1) at 25
0.1) at 25  C. The reacted solution chemistry and mineralogy showed that the mechanism of As(V) sorption was ligand exchange with solid phase SO
C. The reacted solution chemistry and mineralogy showed that the mechanism of As(V) sorption was ligand exchange with solid phase SO in schwertmannite. Two processes presumably occur simultaneously within the reaction period. i.e., ligand exchange of As(V) with surface site SO
in schwertmannite. Two processes presumably occur simultaneously within the reaction period. i.e., ligand exchange of As(V) with surface site SO and subsequent transfer of As(V) to the structure and ligand exchange with tunnel site SO
and subsequent transfer of As(V) to the structure and ligand exchange with tunnel site SO . The exchange ratio between As(V) sorption and SO
. The exchange ratio between As(V) sorption and SO release, and the SO
release, and the SO coordination environment in schwertmannite indicates that monodentate As(V) coordination occurs in surface sites while bidentate binuclear As(V) coordination occurs in tunnel sites. Sorption modeling that considers the different types of reactive sites successfully described the observed As(V) sorption behavior.
coordination environment in schwertmannite indicates that monodentate As(V) coordination occurs in surface sites while bidentate binuclear As(V) coordination occurs in tunnel sites. Sorption modeling that considers the different types of reactive sites successfully described the observed As(V) sorption behavior.
Nakamoto, Tadahiro*; Nakada, Masami; Nakamura, Akio
Journal of Nuclear Science and Technology, 39(Suppl.3), p.102 - 105, 2002/11
no abstracts in English
Tachimori, Shoichi
Nihon Genshiryoku Gakkai-Shi, 42(11), p.1124 - 1129, 2000/11
Times Cited Count:1 Percentile:81.71(Nuclear Science & Technology)no abstracts in English
Sasaki, Yuji; Sugo, Yumi; Tachimori, Shoichi
Proceedings of International Conference on Scientific Research on the Back-end of the Fuel Cycle for the 21st Century (ATALANTE 2000) (Internet), 6 Pages, 2000/10
no abstracts in English
Oda, Chie; *
JNC TN8400 98-001, 14 Pages, 1998/11
Solubilities of amorphous stannic oxide, SnO (am) in Na-ClO
(am) in Na-ClO -Cl-SO
-Cl-SO aqueous systems were measured to quantitatively investigate the influences of the ligands OH
aqueous systems were measured to quantitatively investigate the influences of the ligands OH , Cl
, Cl and SO
and SO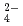 on solubilities. They were also measured in bentonite equilibrated solutions to discuss the behavior of tin under a repository condition of a high-revel radioactive waste. The solubility data in sodium perchlorate media in the range of pH from 6 to 11 showed pH dependency, and the hydrolysis constants of tin (IV) were determined (Amaya, et al., 1997). No significant changes in solubilities with the variation in Cl
on solubilities. They were also measured in bentonite equilibrated solutions to discuss the behavior of tin under a repository condition of a high-revel radioactive waste. The solubility data in sodium perchlorate media in the range of pH from 6 to 11 showed pH dependency, and the hydrolysis constants of tin (IV) were determined (Amaya, et al., 1997). No significant changes in solubilities with the variation in Cl , SO
, SO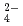 concentrations were observed in Na-ClO
concentrations were observed in Na-ClO -Cl-SO
-Cl-SO aqueous systems, so this indicates that chloride and sulfate species were less effective than hydroxide complexes. On the other hand, solubilities in bentonite equilibrated solutions were higher than solubilities of other experiments in simple systems. These results suggest that (1) other complexes of tin except hydroxide, chloride or sulfate complexes of tin (IV) may dominantly exist in aqueous phase, (2)solid phase other than SnO
aqueous systems, so this indicates that chloride and sulfate species were less effective than hydroxide complexes. On the other hand, solubilities in bentonite equilibrated solutions were higher than solubilities of other experiments in simple systems. These results suggest that (1) other complexes of tin except hydroxide, chloride or sulfate complexes of tin (IV) may dominantly exist in aqueous phase, (2)solid phase other than SnO (am) may limit the solubility of tin under repository conditions.
(am) may limit the solubility of tin under repository conditions.
Saeki, Masakatsu; Nakada, Masami; Yamashita, Toshiyuki; Nakamoto, Tadahiro*; Krot, N. N.*
Radiochimica Acta, 80(2), p.89 - 94, 1998/00
no abstracts in English
Tachimori, Shoichi; Yaita, Tsuyoshi; Suzuki, Shinichi
Proc. of Workshop on Long-Lived Radionuclide Chemistry in Nuclear Waste Treatment, p.179 - 188, 1997/00
no abstracts in English